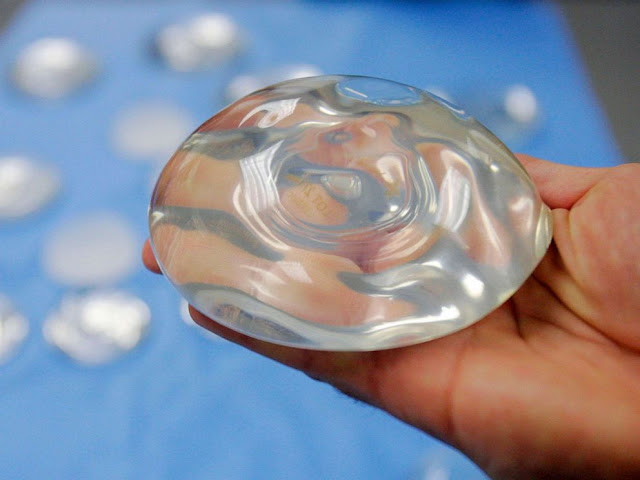
New Breast Implant Guidance From FDA Wants Stronger Warning About Risks Including Cancer The Food and Drug Administration issued new breast implant guidance on Wednesday, recommending that manufacturers include serious warning labels about the potential complications and risks.
The agency’s draft guidance advised that manufacturers lay out risks relating to symptoms like joint pain and fatigue, as well as a link between breast implants and a rare cancer of the immune system, in a boxed warning, also known as a “black-box warning,” that would appear on product labels.
The warning also would list the devices' association with a rare form of lymphoma and say some patients have reported fatigue, muscle aches and joint pain.
The draft guidance, Breast Implants Certain Labeling Recommendations to Improve Patient Communication, provides recommendations for the form and content for certain labeling information for saline and silicone gel-filled breast implants, including:
- Boxed warning
- Patient decision checklist
- Materials/device descriptions, including types and quantities of chemicals and heavy metals found in or released by breast implants
- Silicone gel-filled breast implant rupture screening recommendations
- Patient device card
The recommendations, which have now been put forward for public review, come months after officials linked certain implants to hundreds of U.S. cases of breast implant-associated anaplastic large cell lymphoma, or BIA-ALCL, which is a rare form of cancer that affects the immune system.
Breasts can change for a variety of reasons, including pregnancy, nursing, weight changes and hormonal changes like menopause, added Herluf Lund, a St. Louis-based plastic surgeon and member of the American Society for Aesthetic Plastic Surgery, in a statement sent to MarketWatch.
"We have heard from many women that they are not fully informed of the risks when considering breast implants," FDA Principal Deputy Commissioner Amy Abernethy and Jeff Shuren, director of the agency's Center for Devices and Radiological Health, said in a statement.
A boxed warning is one of the FDA’s most serious warnings. It typically appears in a bold font in black box on prescription drug labels and is designed to “call attention to serious or life-threatening risks,” according to the agency.
The devices are used in about 400,000 surgeries in the United States every year, with 75 percent of the women involved getting implants for cosmetic reasons. Most of the rest get them as part of reconstruction after surgery for breast cancer.
Once the guidance is finalized, manufacturers will have the option to either follow the recommendations or choose other methods of labeling, "so long as the labeling complies with applicable FDA laws and regulations," it added.
New Breast Implant Guidance From FDA Wants Stronger Warning About Risks Including Cancer
 Reviewed by Prince2030
on
2:56:00 PM
Rating:
Reviewed by Prince2030
on
2:56:00 PM
Rating:
 Reviewed by Prince2030
on
2:56:00 PM
Rating:
Reviewed by Prince2030
on
2:56:00 PM
Rating:



Oh its really nice, i like it. Thanks for sharing this information. if anyone has the need of Breast Augmentation in Vizag, india, must visit the Dr. VJs Cosmetic Surgery & Hair Transplantation Centre.You must contact on 9849797776 and must visit the official site.
ReplyDeleteWant to get done your breast implant surgery with Dr. Amit Gupta? Don't wait go ahead and book your appointment on 9811994417. Get your breast enhancement in Delhi with very effective way. He has done 1700 accomplished procedures.
ReplyDelete